Our Research

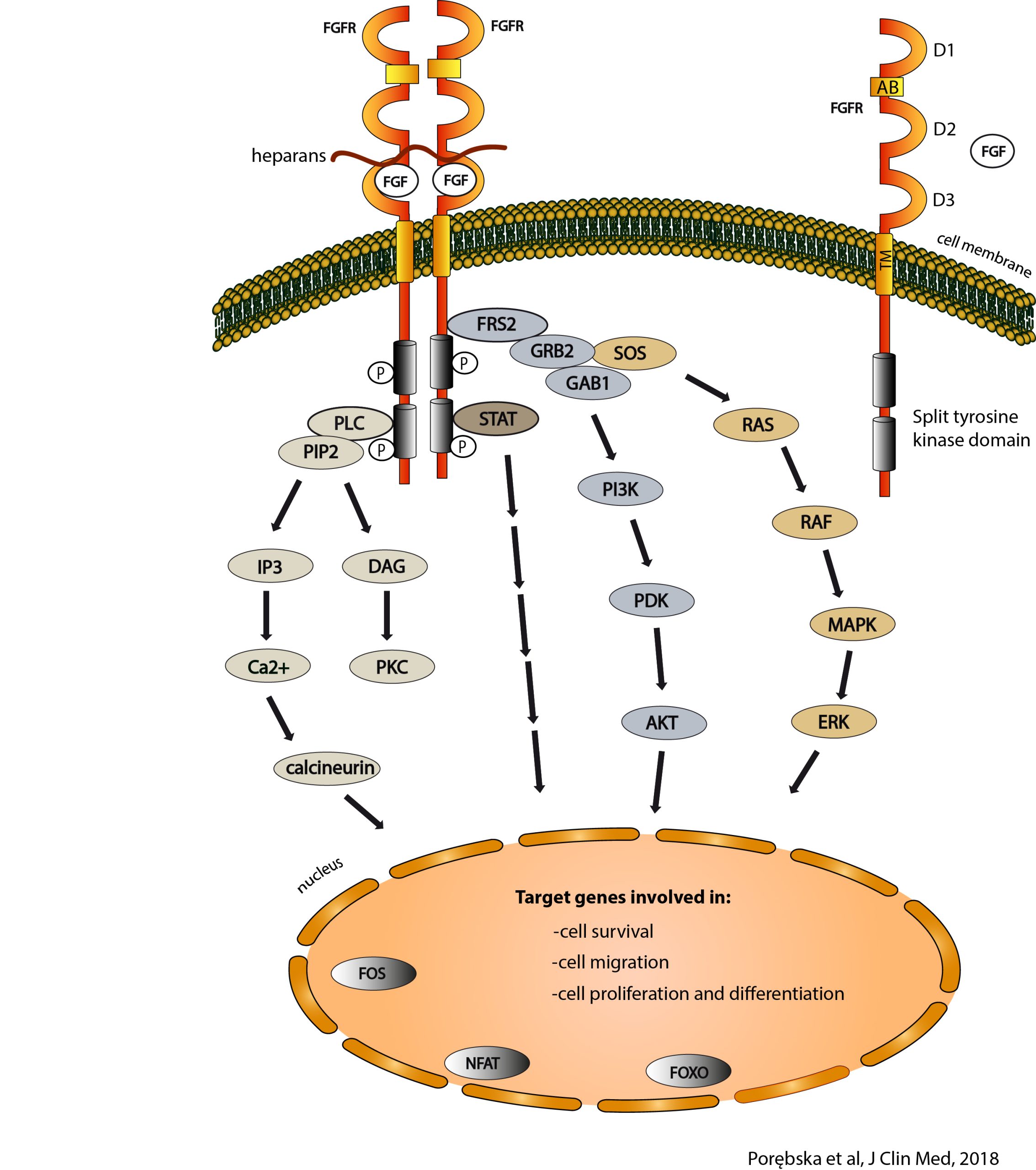
Fibroblast growth factors FGF (Fibroblast Growth Factors) and their receptors FGFR (Fibroblast Growth Factor Receptors) are signaling hubs that enable the transmission of signals from outside the cell to its interior. Extracellular growth factors FGFs recognize FGFRs anchored in the cell membrane, influencing their conformation and leading to the activation of their intracellular domains with tyrosine kinase (TK) activity. This, in turn, leads to the initiation of intracellular signal transduction cascades that regulate basic cellular processes such as apoptosis, motility, metabolism and division. The FGF/FGFR system is necessary for the proper development of the human body, and its defects are associated with a number of diseases, including cancer. In approximately 10% of cancers, changes in FGF/FGFR are observed, which usually lead to uncontrolled, excessive activation of FGF/FGFR-dependent signaling pathways, facilitating tumorigenesis.
In our research, we focus on identifying proteins of the extracellular space and the plasma membrane that can regulate signaling through the FGF/FGFR system. We also investigate FGFR oligomerization and the influence of different oligomeric states of FGFR on the specificity of signal transduction.
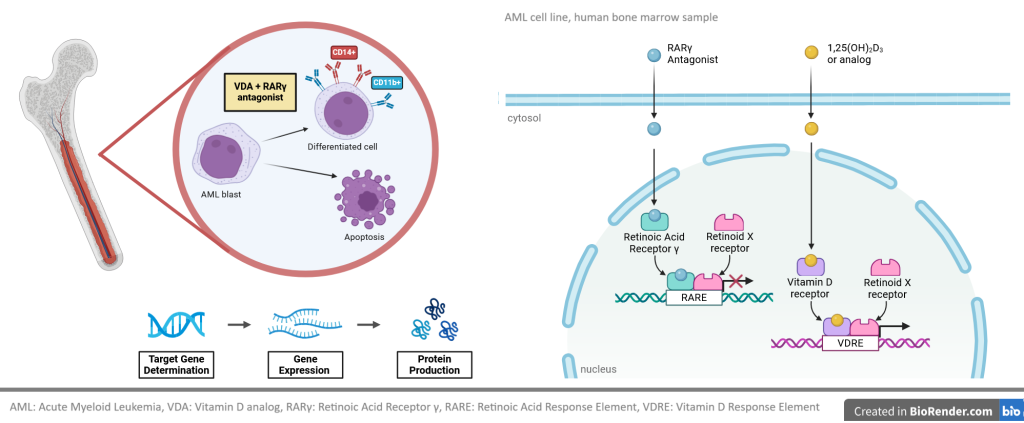
In our research we want to determine whether the combination of retinoic acid receptor (RAR) γ antagonist and vitamin D receptor (VDR) agonist are suitable as a differentiation therapy against acute myeloid leukemia (AML). We will determine the affinities of RARγ antagonist, VDR agonist, and of new hybrid compounds to recombinant RARγ and to recombinant VDR. We will also investigate how these compounds activate transcription of VDR-target genes and how they block transcription of RARγ-target genes. We will study activities of the above compounds against AML cell lines and against AML bone marrow blasts from patients. Eventual goal will be to determine global transcriptome in AML cell lines and AML blasts exposed to RARγ antagonist ± VDR agonist.
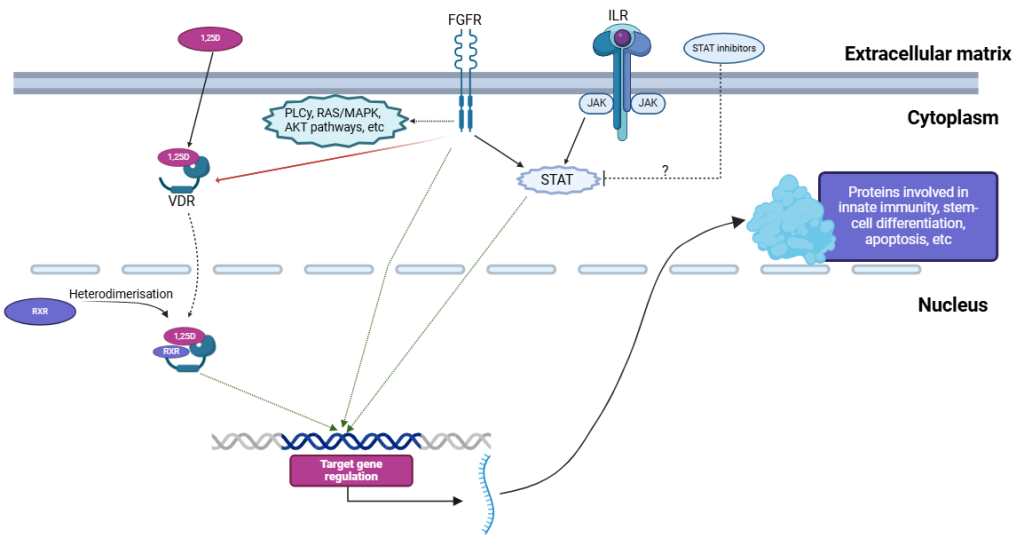
Vitamin D receptor (VDR) is present and active in many blood cells, and the correct level of vitamin D is necessary for proper function of the immune system. Active metabolite of vitamin D, 1,25-dihydroxyvitamin D (1,25D) supports differentiation of monocytes and innate immune functions. 1,25D, and its analogues are able to induce differentiation and to suppress proliferation in these acute myeloid leukemia (AML) cells which express VDR and have sufficient level of VDR protein.
Our studies revealed that aberrant signal transduction from constitutively active fusion protein FOP2-FGFR1 is responsible for downregulation of expression of VDR gene in AML cells, which results in resistance to 1,25D-induced differentiation. On the other hand, too many wild type FGF receptors on leukemia cells sensitize them to 1,25D. Now, we are investigating which intracellular signaling pathways might be employed to enhance differentiation therapy of AMLs.
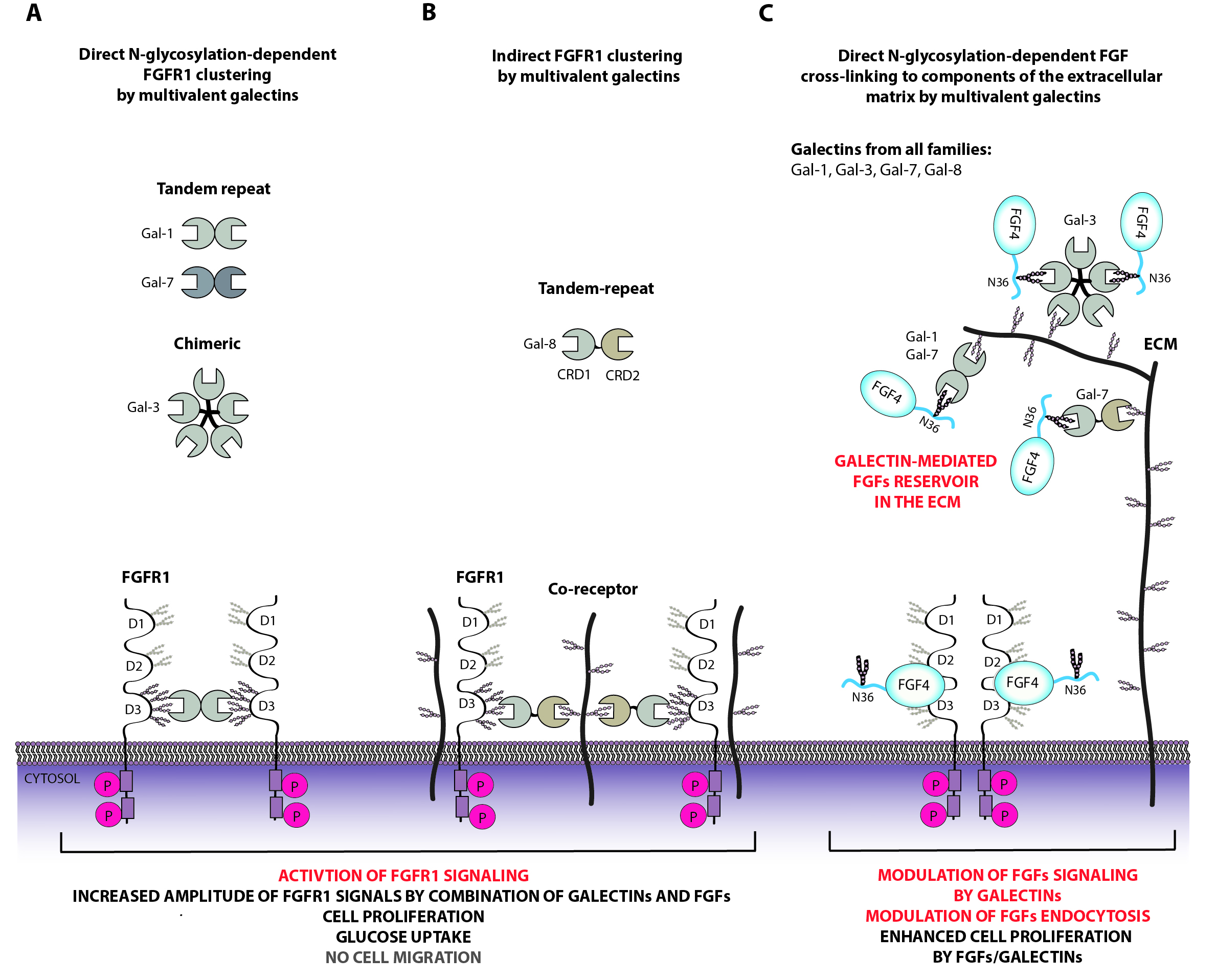
Galectins are lectins that specifically recognize sugars, in particular terminal galactose on sugar chains attached to proteins or lipids. Galectins are involved in many key cellular processes (including signal transduction, endocytosis, gene expression, intercellular contacts), they also play an important role in the invasion of human cells by pathogens, in the immune response and in the process of carcinogenesis. Galectins are composed mainly of the carbohydrate recognition domain CRD, to which additional structural elements may be attached. Galectins, based on their molecular architecture, are classified into three subfamilies: prototypical galectins (galectins containing a single CRD, capable of dimerization), tandem galectins (galectins containing two different CRDs on one polypeptide chain, also capable of oligomerization) and chimeric galectins (galectin-3 belongs to this family, which, in addition to the CRD, has a long unstructured N-terminus).
At the Department of Medical Biotechnology, we study the functions of galectins both on the cell surface and inside the cell, mainly in the context of transmitting signals through receptors with the tyrosine kinase activity (RTK) and their ligands, and study their role in endocytosis of surface receptors. We investigate how galectins read the sugar code stored in the structure of glycoproteins for signal transduction. We also investigate the importance of the specificity and multivalency of galectins, and their ability to undergo liquid-liquid phase transitions (LLPS) for their functions.
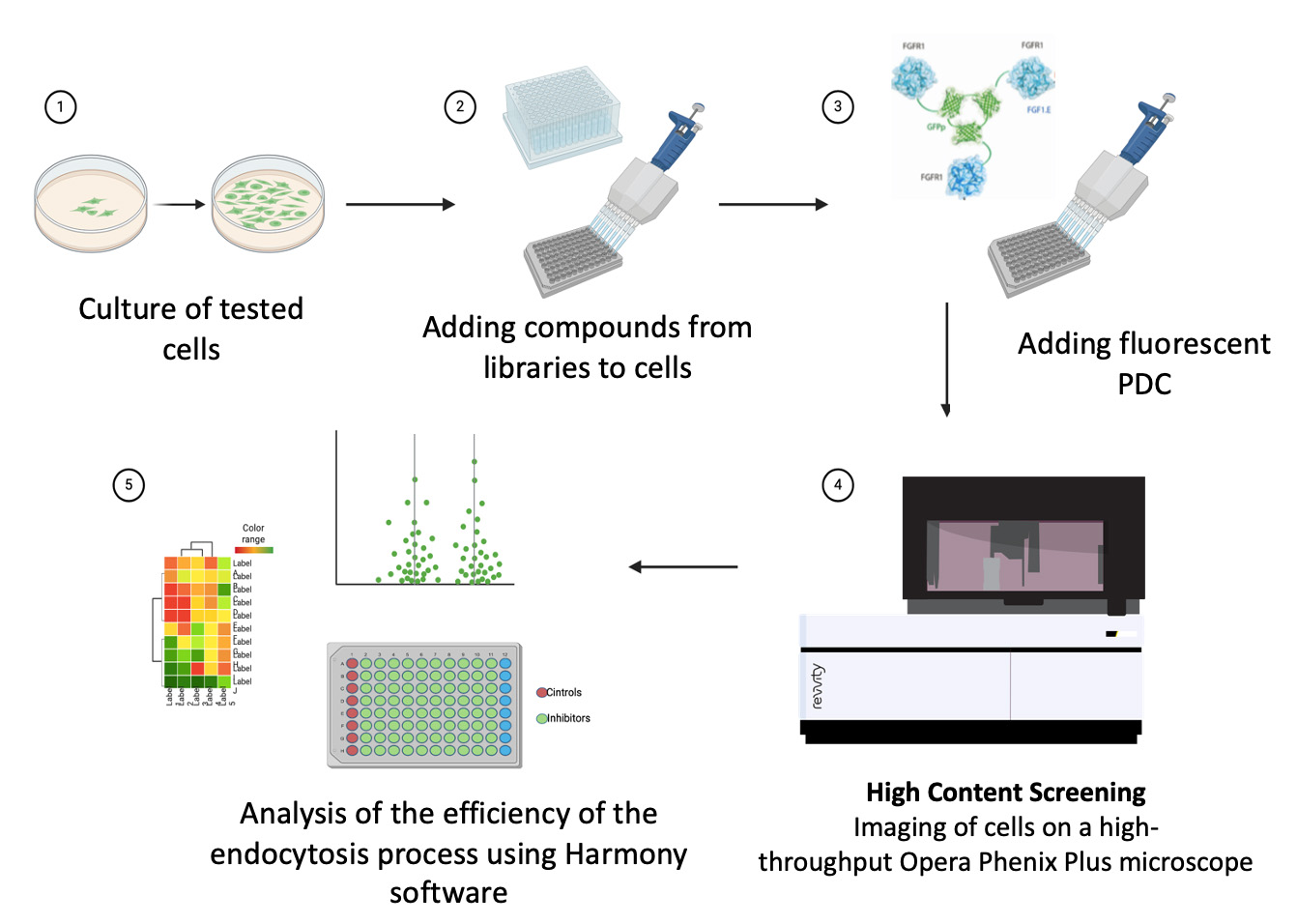
Endocytosis is a set of molecular pathways that, using different mechanisms, enable a cell to take up substances from its surface and its environment. Endocytosis seems to be under strict control of cellular signaling pathways, but the connections between these complex processes are still unclear.
In the Department of Medical Biotechnology, we use the high-throughput imaging technique HCS (High Content Screening) and large, representative compound libraries (e.g. a kinase inhibitor library containing over 2000 compounds) to identify specific signaling pathways regulating the studied cellular process, mainly endocytosis. The obtained basic knowledge, apart from being crucial for understanding cellular mechanisms, allows us to modulate the delivery of cytotoxic drugs in a targeted anticancer approach such as PDC (Protein Drug Conjugates).
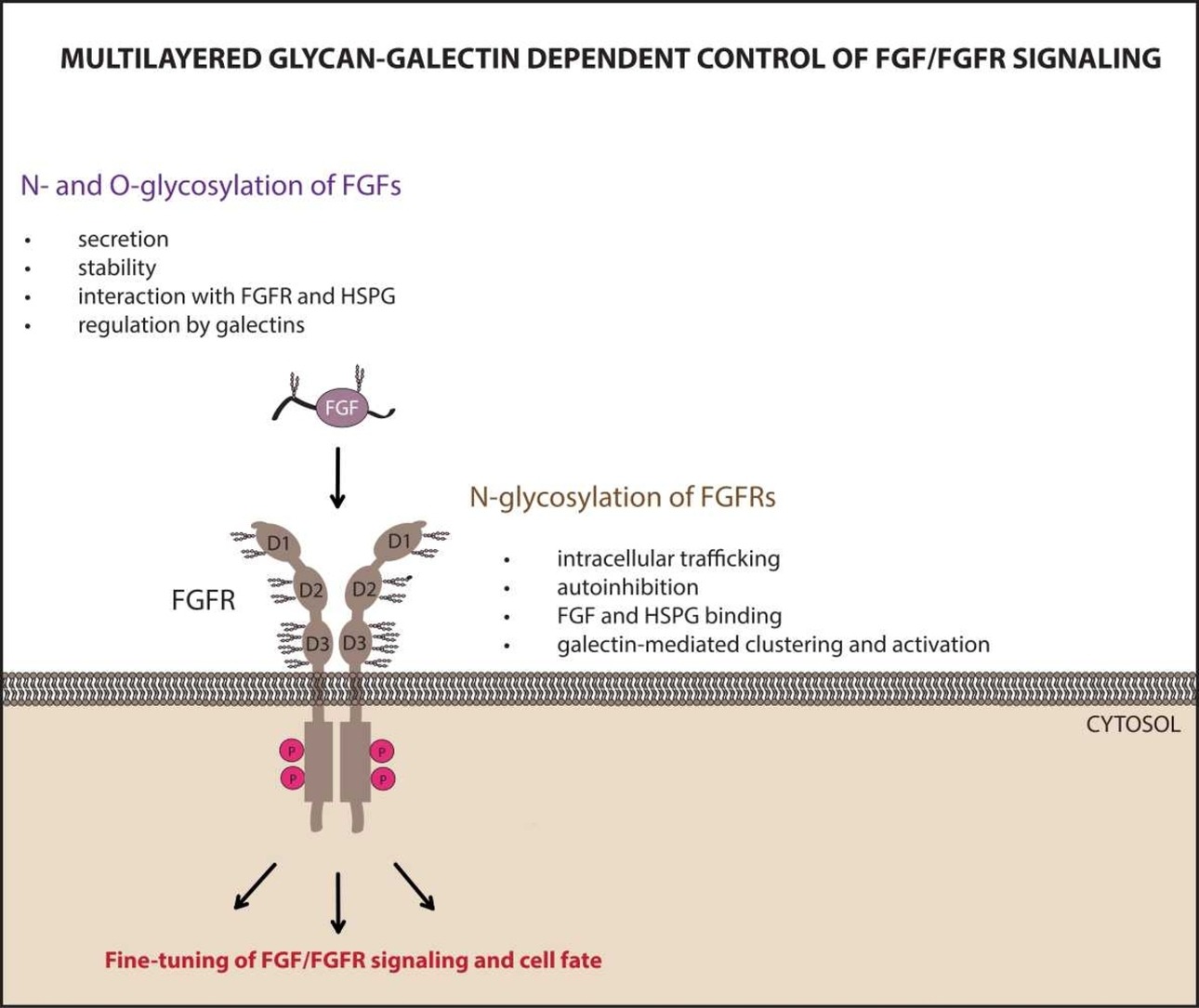
Both FGFR receptors and the vast majority of FGF proteins are modified by attaching complex sugar structures to polypeptide chains. In the case of FGFR receptors, a significant part of their extracellular region is covered by N-glycans. In the case of FGF proteins, we are dealing with N- and O-glycosylation, usually at one or two positions; these are typically unstructured regions of FGF proteins, which may constitute the uniqueness of FGF proteins. Despite individual literature reports suggesting an important role of glycosylation for the operation of the FGF/FGFR system, the exact significance of these modifications is unknown.
At the Department of Medical Biotechnology, we study post-translational modifications of FGF and FGFR, focusing on N-glycosylation of these proteins. In our work, we determine the importance of FGF/FGFR N-glycosylation for intermolecular interactions, stability, activation and cellular transport of these proteins. We also investigate the role of FGF/FGFR glycosylation as a “glycocode” on the cell surface read by lectins from the galectin group.
With almost 20 million new cases per year, cancer is one of the most important challenges facing public health systems. In contrast to traditional chemotherapy, targeted cancer strategies use advanced therapies to precisely identify and target cancer cells, limiting the impact of drugs on healthy cells and thus minimizing unwanted side effects of therapy. Protein Drug Conjugates (PDCs) are a rapidly growing group of targeted therapies consisting of a protein receptor-recognizing agent specific to cancer cells covalently conjugated to a highly potent cytotoxic drug. Several PDCs, mainly in the form of Antibody Drug Conjugates (ADCs), which use monoclonal antibodies as cancer recognition molecules, are used in the clinic, and many PDCs are currently in clinical trials.
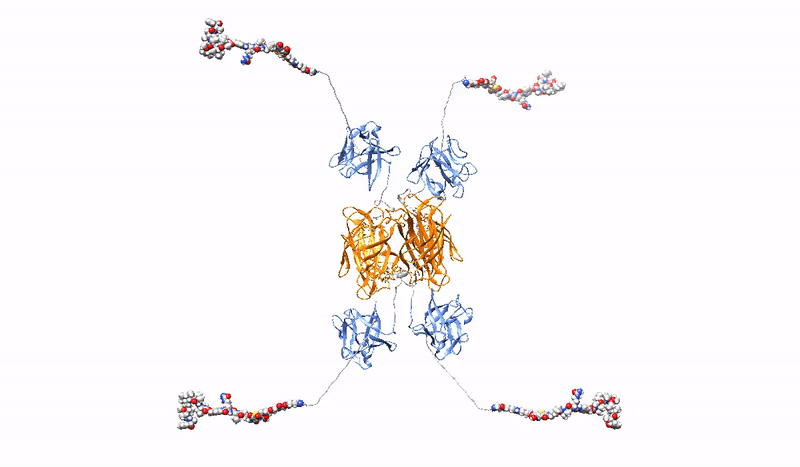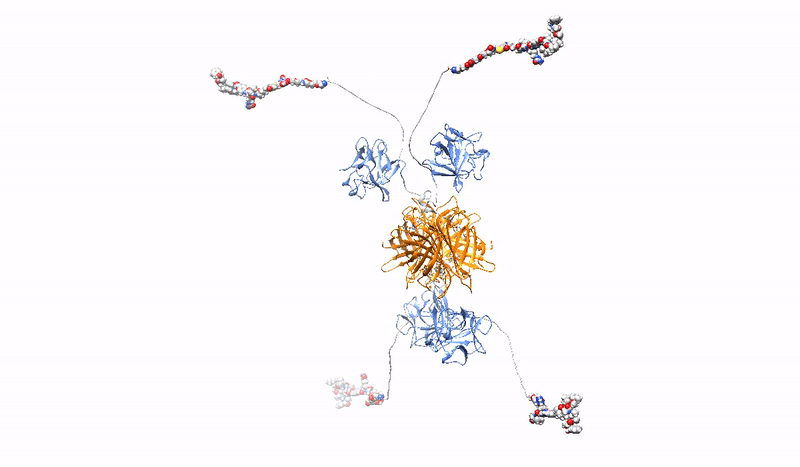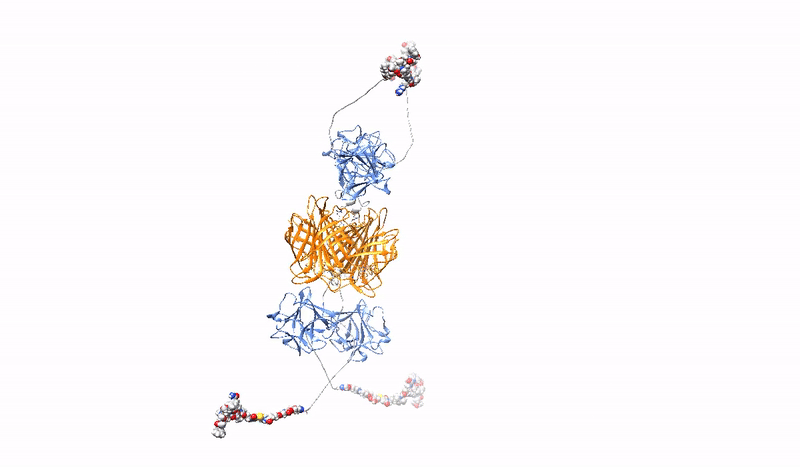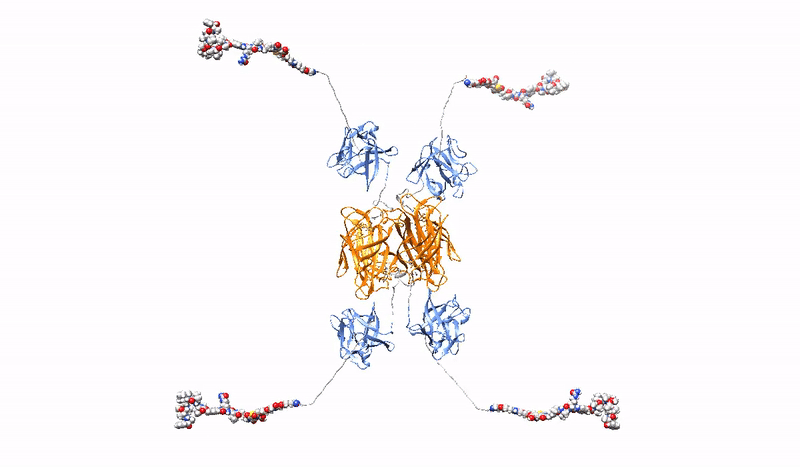
Highly selective, strong and stable interaction of PDC with a tumor marker, combined with efficient, rapid endocytosis of the receptor/PDC complex and its subsequent effective delivery to lysosomes, is crucial for the effectiveness of targeted cancer therapy with PDC. However, the bivalent architecture of monoclonal antibodies, on which modern PDCs are based, is not optimal.
At the Department of Medical Biotechnology, we develop multivalent (capable of binding multiple oncogenic receptors simultaneously) PDCs, which are characterized by increased precision and efficiency of drug delivery to tumor cells. We construct multivalent PDCs with different architecture, methods for site-specific drug attachment, specificity and various additional features (e.g. stable fluorescence). Due to stronger and more stable binding, our multivalent PDCs are able to cross-link oncogenic receptors on the surface of cancer cells, leading to the simultaneous induction of several endocytic pathways or aggregation-dependent endocytosis (ADE) route, which ensures significantly better drug uptake and efficient targeting to lysosomes to release the toxic drug inside the cancer cell, causing its death.
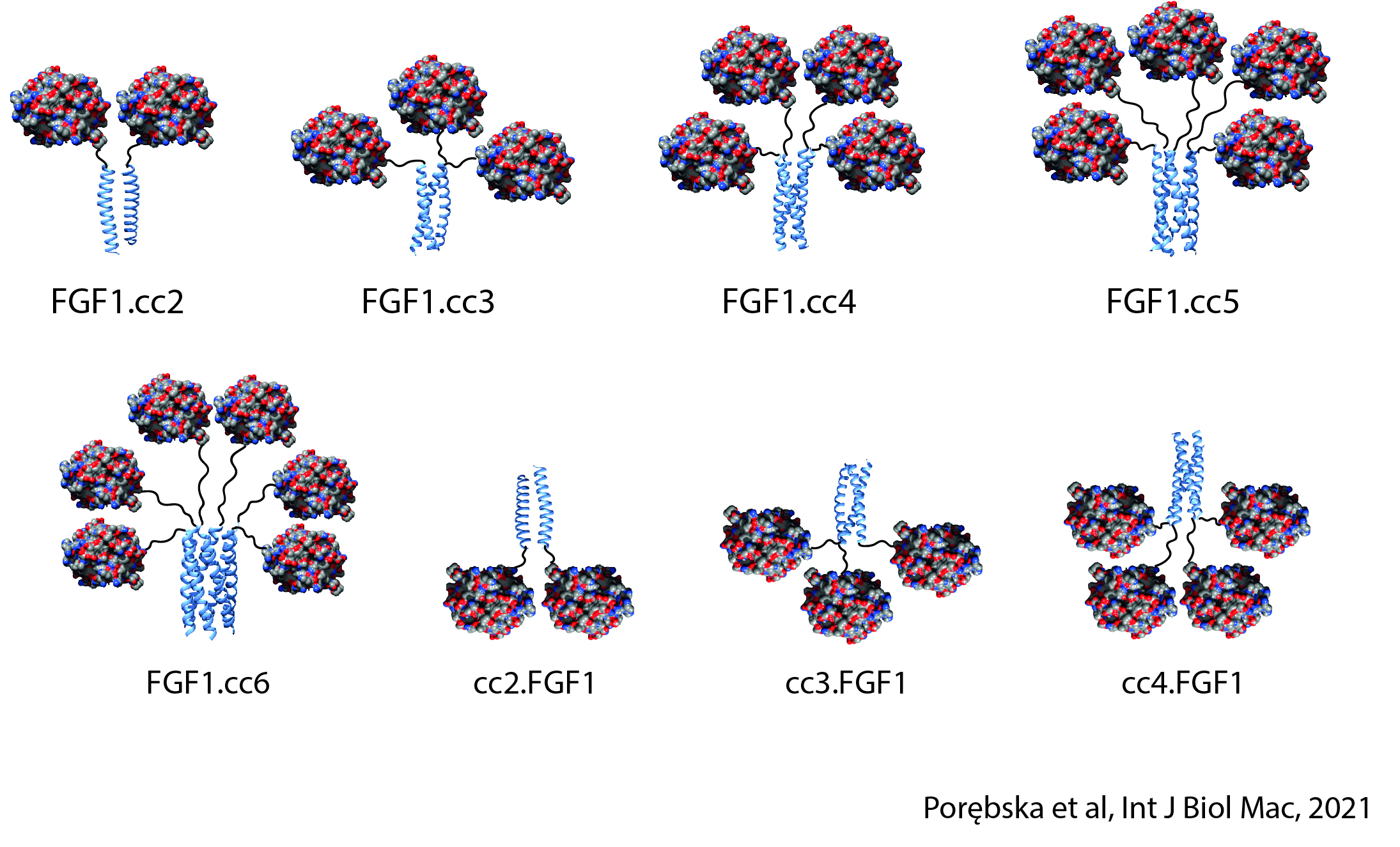
The strength and specificity of the signals transmitted by cell surface receptors may determine the final effect of the signals on cell behavior (e.g., determine the switch from steady state to division). At the Department of Medical Biotechnology, we test whether the strength and specificity of signals transmitted by receptors with RTK tyrosine kinase activity depend on the oligomeric state of the receptors. We design and prepare oligomeric and mutational variants of galectins and growth factors, which are characterized by changed properties in relation to the starting proteins, e.g. increased affinity for receptors, the ability to cross-link receptors, the ability to modulate receptor endocytosis, changed specificity or improved stability. The obtained proteins can be used as drug carriers, metabolism modulators or factors stimulating wound healing.
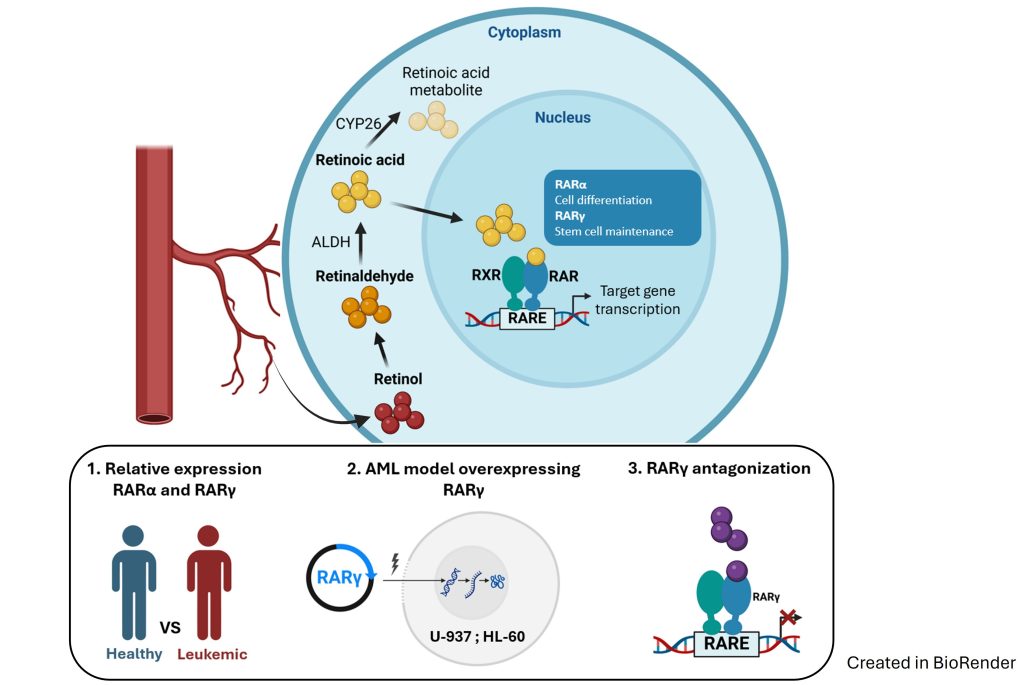
In our research we aim to determine the roles of retinoic acid receptors (RAR) α and γ in the development of acute myeloid leukemia (AML). We investigate the relative expressions of RARA and RARG in bone marrow leukocytes from healthy people and in blasts from patients with preleukemic states and with leukemias. We aim to prepare AML cell line models with overexpression of RARG in order to determine how it affects its malignant properties. Eventually, we will test the activity of RARγ antagonist against the above AML cell lines, and against bone marrow blasts from patients.
Cells maintain a functional proteome by precisely balancing the content, concentration, conformation, and subcellular localization of proteins. This requires a coordinated interaction between sophisticated cellular molecular machines that support proteins from the moment of their synthesis to the final moments of protein life, seen as loss of function and subsequent degradation. Failure to maintain protein homeostasis has dramatic consequences for cells and can ultimately lead to pathophysiological conditions or cell death. Since dysregulated proteins are critical drivers of most human diseases, including cancer, they represent molecular targets for various therapeutic interventions.
Although more than 4000 proteins have been identified that are associated with human diseases, only about 10% of them have been successfully exploited in therapeutics to date. Therefore, it is necessary to develop new strategies that enable the targeted inactivation of numerous disease-causing proteins that are beyond the reach of current methods. About two decades ago, the concept of Targeted Protein Degradation (TPD) was developed. TPD engages the cells’ natural proteolytic mechanisms (primarily proteasomes or lysosomes) to specifically remove disease-related proteins from the cell, targeting proteins that have so far been beyond the reach of conventional drug development methods.
At the Department of Medical Biotechnology, we are developing new methods for targeted protein inactivation in the cell that, unlike current TPD strategies, do not rely on autophagy and proteasomal degradation.