Our Research Projects


PROT-TOWING: Development of an Innovative Molecular Towing Technology for the Selective Inactivation of Pathogenic Proteins
Project objective:
Dysregulated pathogenic proteins (POIs) are the causative agents of many human diseases and constitute molecular targets for therapeutic interventions. Traditionally, pathogenic POIs are inhibited by low-molecular-weight enzyme inhibitors or ion channel blockers, which constitute the majority of currently used drugs. Despite the undoubted success of conventionally developed drugs, this approach has proven effective for only about 10% of pathogenic proteins, and most pathogenic POIs (e.g. transcription factors, building block proteins) are currently beyond our reach.
The aim of the PROT-TOWING project is to develop an innovative technology for the targeted inactivation of pathogenic proteins in the cell, including molecular targets inaccessible to current therapeutic approaches, which may enable the development of therapeutic strategies for previously incurable diseases in the future.
As part of the research project chemistry, biophysics, biochemistry, molecular and cellular biology techniques will be used. Advanced research equipment in the form of a biolayer interferometer (BLI) and a low-temperature freezer will be purchased to implement the project.
The result of the project will be a new technology enabling selective inactivation of cytosolic proteins PROT-TOWING.
The groups interested in project outcome are biotechnological, chemical or pharmaceutical companies, and in the future possibly patients (in the event of a future application for the PROT-TOWING technology for drug development). The project will also result in the development of new scientific staff.
We will cooperate with the domestic economic partner – Captor Therapeutics S.A. and with the foreign scientific partner – prof. Ida van der Klei from the University of Groningen.
The project is financed from European Union funds.
PI (main contractor): prof. dr hab. Łukasz Opaliński
Project value: PLN 3,878,448.40
European Funds contribution: PLN 3,878,448.40
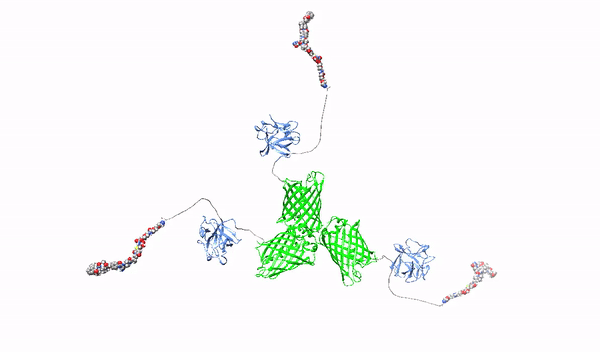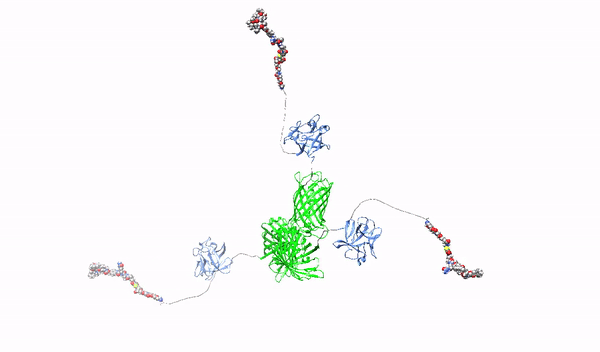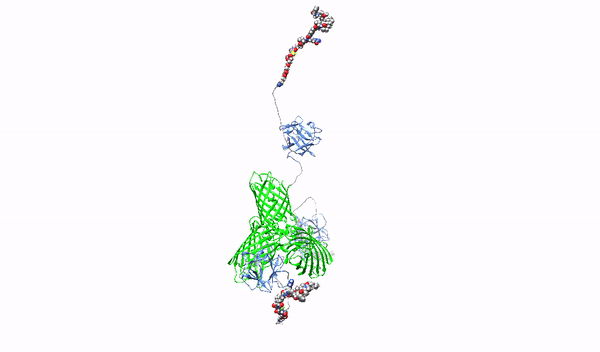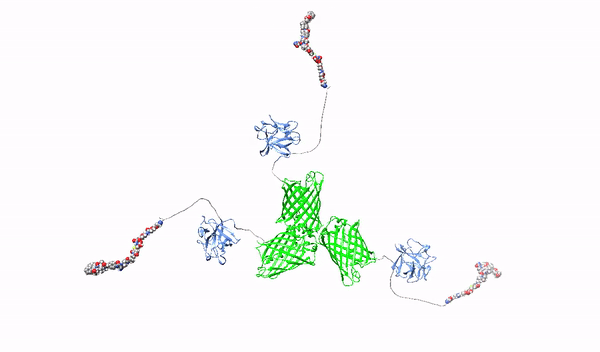
Targeting surface proteoglycans with high-affinity multimeric ligands and their cytotoxic conjugates – towards precision therapy for pancreatic cancer
NCN Nr 2021/43/B/NZ1/00245 (2022-2026); PI: prof. dr hab. Łukasz Opaliński
Pancreatic cancer is the most deadly cancer (5-year survival after diagnosis is only 9%), and the number of diagnosed patients is constantly increasing. The high mortality rate of pancreatic cancer results from late diagnosis, limited possibilities of surgical intervention and limited availability of effective therapeutics. Therefore, new methods of diagnosis and possibly non-invasive treatment of pancreatic cancer are being intensively sought, in particular approaches based on precision cancer medicine, which is characterized by significantly fewer side effects compared to conventional therapies, e.g. chemotherapy.
Cancer cells are characterized by increased frequency and uncontrolled nature of division, intensive migration and avoidance of death. Proteoglycans (HSPGs) are cell surface proteins that, by organizing the action of growth factors and their receptors, enable cells to acquire the above oncogenic activities. Importantly, significantly increased amounts of HSPGs have been observed on the surface of pancreatic cancer cells compared to healthy cells. Differences in HSPGs levels between healthy and cancerous pancreatic cells and the involvement of HSPGs in the process of carcinogenesis provide the basis for designing targeted anti-cancer therapies.
This project will develop novel molecules recognizing HSPGs with high affinity, which will serve as factors blocking the oncogenic effect of HSPGs and as carriers of cytotoxic drugs for selective killing of pancreatic cancer cells. As a starting molecule, we will use the first fibroblast growth factor, FGF1, which has a natural ability to interact with HSPGs. The FGF1 protein will be reprogrammed in such a way that it loses its natural ability to stimulate cell division and its ability to bind HSPGs will be improved. Then, using such modified FGF1 and various molecular scaffolds, FGF1 oligomers, HMLAs, will be produced, specifically and very strongly interacting with HSPGs. HMLAs, by binding HSPGs, will block the oncogenic action of growth factors via HSPGs and serve as drug carriers to selectively kill pancreatic cancer cells overproducing HSPGs.
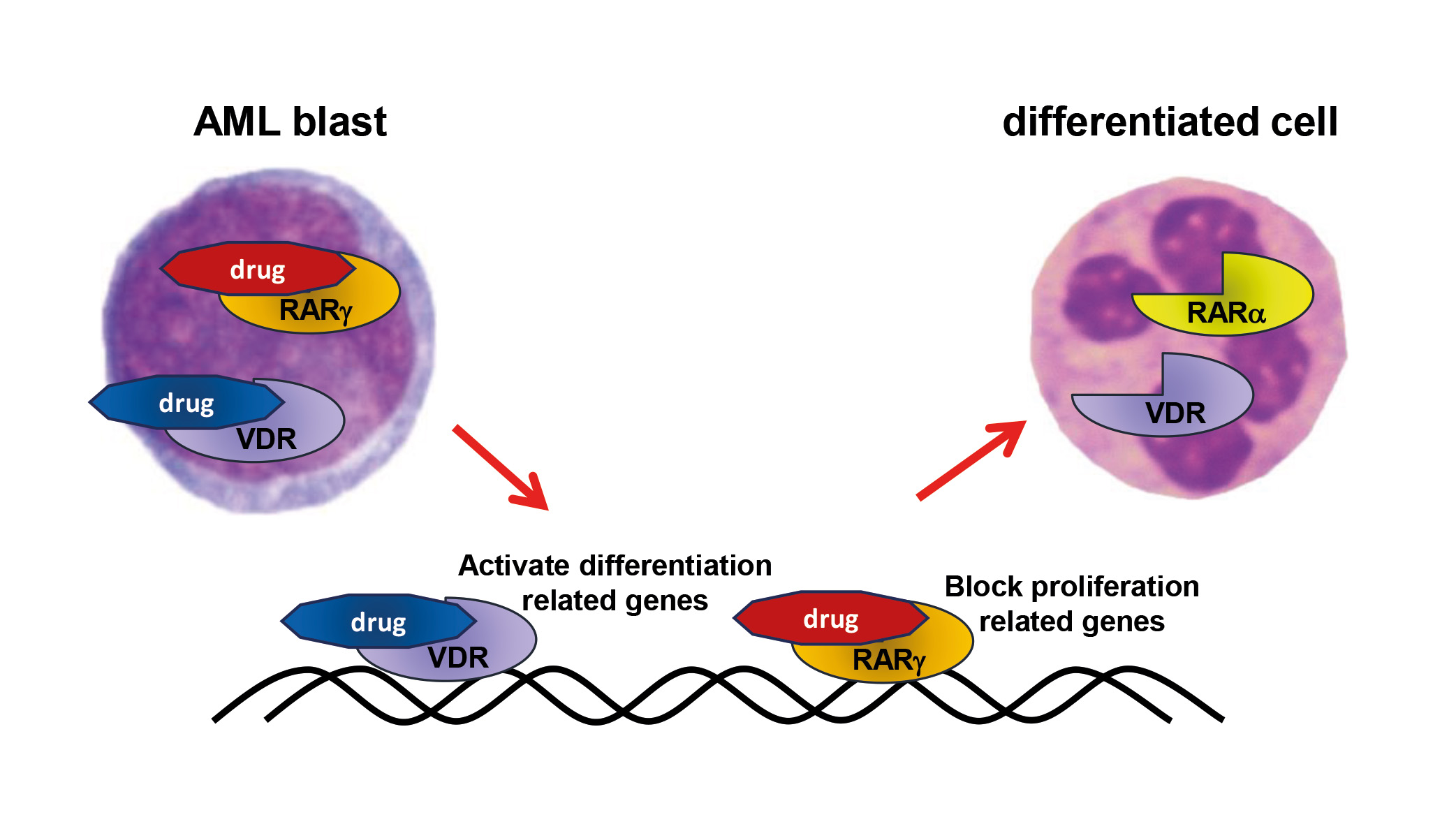
Innovative ligands for nuclear receptors to eradicate cancer relapse – eRaDicate
Marie Skłodowska-Curie Doctoral Networks (MSCDN); Call: HORIZON-MSCA-2022-DN-01-01, PI: prof. dr hab. Ewa Marcinkowska
The aim of the international anticancer drug research and development program “eRaDicate” is to enable 11 young scientists to advance in cancer research, while developing novel therapies against relapses and metastases caused by cancer stem cells. Nuclear receptors, such as the retinoic acid receptor (RAR) and the vitamin D receptor (VDR), play an important role in some cancers and can be effective targets for anticancer therapies. To address the EU priority “Mission Cancer”, we will
1) test the anticancer activity of compounds targeting RAR and VDR;
2) design and synthesize a dual-acting hybrid compound acting simultaneously as an RARγ antagonist and a VDR agonist;
3) develop pre-formulation strategies for our compounds;
4) develop a novel deep learning-based method for cancer analysis and diagnostics.
The group from the University of Wrocław, which is part of the “eRaDicate” consortium, is researching the anti-leukemic activity of new VDR and RARg receptor ligands, the mechanisms of their action and the role of RARg as an oncogene in blood cells.
Website of the project:
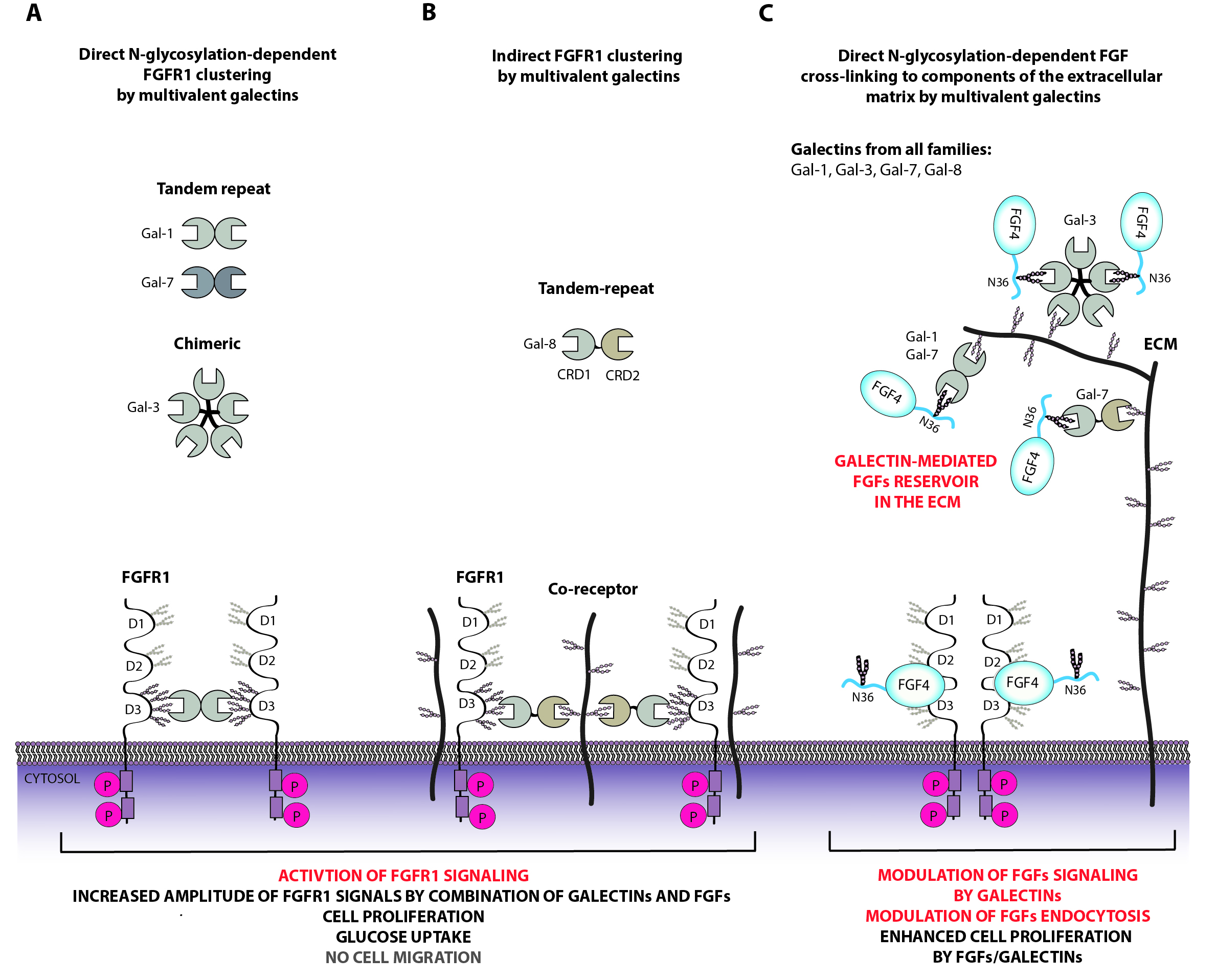
Regulation of fibroblast growth factor receptors by extracellular galectins
NCN Nr 2019/34/E/NZ3/00014 (2020-2025); PI: prof. dr hab. Łukasz Opaliński
Cell function is largely regulated by protein receptors located on the cell surface, which transmit signals from the extracellular space to the interior of the cells. Signal transduction pathways dependent on fibroblast growth factor receptors (FGFR) regulate metabolism, cells, cell differentiation, division and motility. FGFR proteins are crucial for the proper development and functioning of the human body, and uncontrolled changes in FGFR function lead to serious metabolic diseases and cancer. Due to their key role, FGFR proteins are subject to strict and multi-level control in the cell. Recently, we have identified a new mechanism of FGFR regulation. We have shown that extracellular sugar-binding proteins: galectin-1 and galectin-3 interact with sugar residues attached to FGFR proteins, which affects FGFR signaling and cell function. The human galectin family consists of eleven proteins, and their role in FGFR regulation is unknown.
Our research hypothesis assumes that signaling through FGFR receptors and cellular transport of FGFRs is subject to complex regulation by extracellular proteins from the galectin family. The main goal of this project is to understand the role of all proteins from the galectin family in the regulation of cellular transport and function of FGFR receptors. As part of the project, we will determine which galectins interact with FGFRs and characterize these interactions in detail. We will investigate whether and how galectins affect the interaction of FGFR receptors with their partner proteins in the cell. We will determine the role of galectins in the regulation of signaling through FGFRs and in the cellular transport of these receptors. In the final phase of the project, we will investigate how the interdependence of galectins and FGFRs affects cell function.
The results obtained during the implementation of this project will be characterized by a high degree of novelty and great significance for the development of the scientific discipline. Understanding how proteins from the galectin family and FGFR receptors cooperate in the regulation of cell function is crucial for the development of cell biology and understanding the process of carcinogenesis. Additionally, the obtained data may enable the design of new therapies against cancer and metabolic diseases.
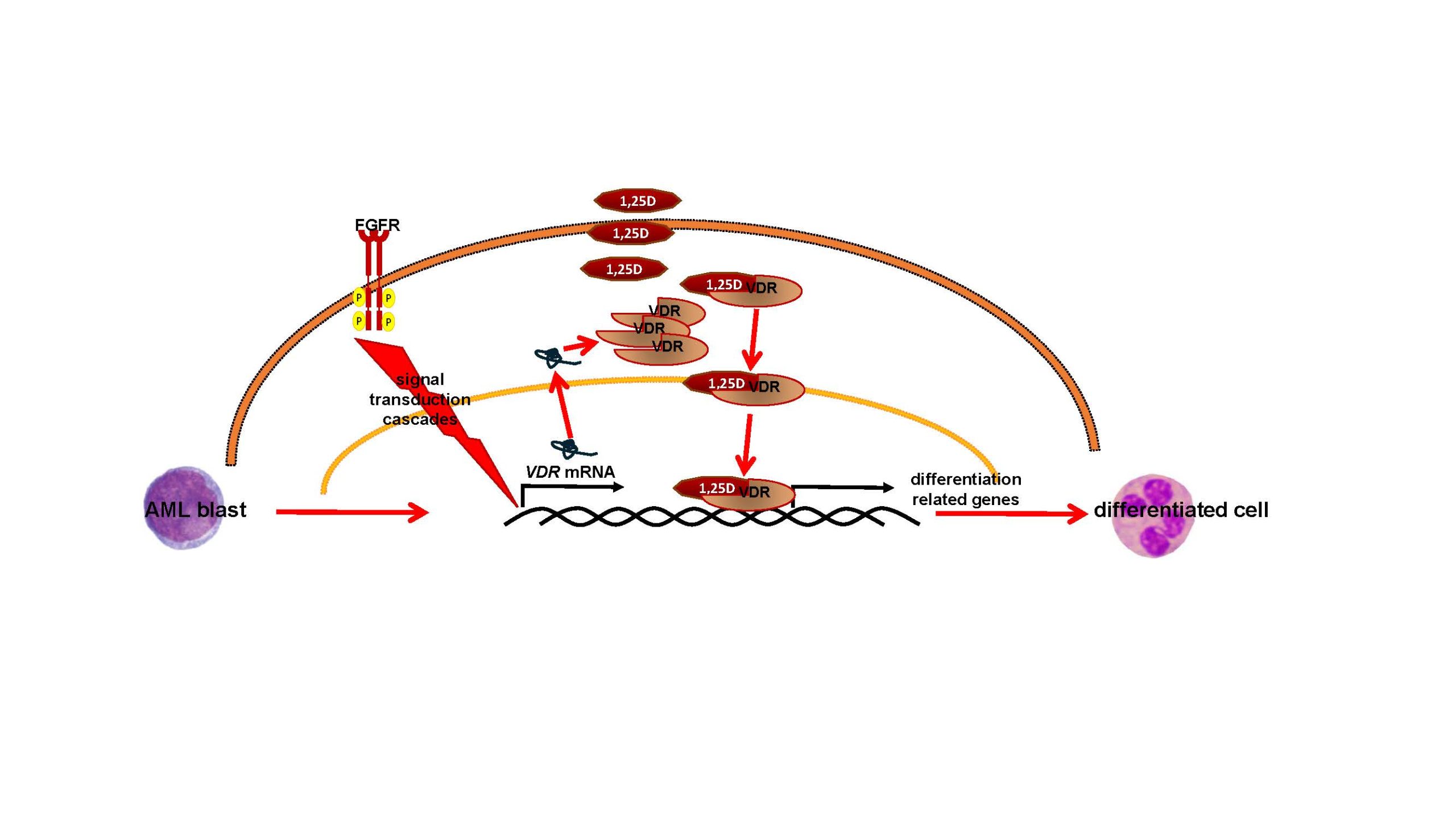
Study of the importance of fibroblast growth factor receptors and STAT transcription factors in targeted therapy of acute myeloid leukemia
NCN Nr 2022/47/O/NZ5/00288 (2023-2027); PI: prof. dr hab. Ewa Marcinkowska
Fibroblast growth factors (FGFs) and their receptors are critical for normal embryonic development and wound healing. However, mutations in the genes that code for FGF receptors can lead to the development of cancers, both solid tumors and leukemias. Some of these mutations are so-called gene amplifications, which cause cells to have too many receptors on their surface. It is not clear how often such amplifications occur in leukemias, because this type of mutation is not routinely diagnosed in patients with these diseases. Recently, our team discovered that too many FGF receptors on leukemia cells make them more sensitive to the active form of vitamin D, called 1,25-dihydroxyvitamin D. We also found that blocking transcription factors called STATs, using an inhibitor called fludarabine, can be beneficial for patients.
The main role of vitamin D, which is produced by the human body from cholesterol under the influence of exposure to sunlight, is to regulate calcium and phosphate metabolism, prevent rickets and osteoporosis. In recent years, it has been proven that 1,25-dihydroxyvitamin D is also important for the development of blood cells and the functioning of the immune system, and a deficiency of vitamin D promotes the development of autoimmune diseases and some cancers. Under the influence of 1,25-dihydroxyvitamin D, the cells of some leukemias begin to resemble normal cells of the immune system. Therefore, we would like to know in how many cases of leukemias there are amplifications of the genes encoding FGF receptors and whether in the case of leukemias with such mutations, therapy with analogues of 1,25-dihydroxyvitamin D and fludarabine could improve the patient’s condition.
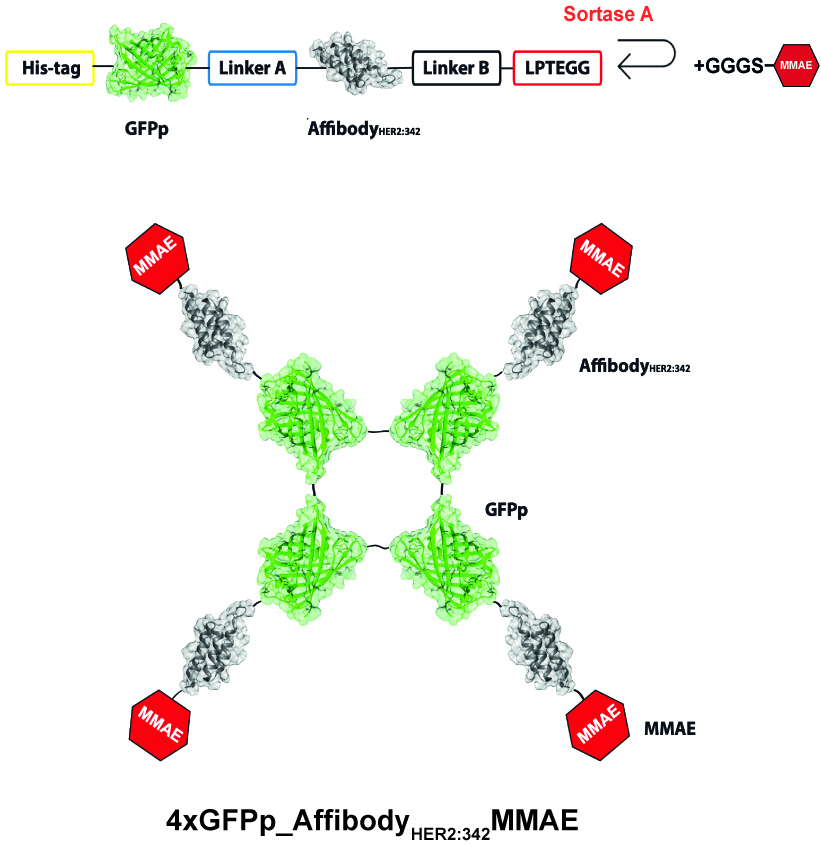
Efficiently internalizing fluorescent oligomeric cytotoxic conjugate targeting HER2-overexpressing breast cancer cells
NCN Nr 2022/45/N/NZ1/00088 (2022-2025); PI: dr Natalia Porębska, scientific supervisor: prof. dr hab. Łukasz Opaliński
Breast cancer is the most common malignant tumor in women. Of all diagnosed cases, approximately 20% are breast tumors characterized by excessive expression of the HER2 receptor on the cell surface. HER2 is currently considered one of the main oncogenic factors in breast cancer. Several therapeutic strategies have been developed to date, which unfortunately show limited efficacy. A major problem is the high cost of current therapies, as well as the ability of cancer cells to acquire resistance to drugs. The development of effective therapies against HER2+ breast cancer is still a major challenge for modern medicine. One of the therapeutic approaches developed so far in the treatment of breast cancers with HER2 overexpression are antibody conjugates with a cytotoxic drug. An important feature determining the effectiveness of such therapies is the specific and efficient penetration of cytotoxic conjugates via the HER2 receptor into the cancer cells, where the active drug is released. Unfortunately, HER2 is a receptor characterized by poor penetration into the cells, which is why new strategies are desirable to increase the efficiency of this process.
Based on our previous observations, we hypothesize that cross-linking the HER2 receptor into larger complexes on the cell surface via oligomeric cytotoxic conjugates will enhance the penetration of the drug into cancer cells via HER2. The aim of the proposed project is therefore to develop a new oligomeric cytotoxic conjugate that effectively eliminates cancer cells overexpressing the HER2 receptor. An additional advantage of the designed conjugate will be its fluorescence, which will enable monitoring of the distribution and action of the cytotoxic drug.

Liquid-liquid phase separation as a mechanism of biological activity of human galectins
NCN Nr 2023/07/X/NZ1/01396 (2023-2024); PI: dr Marta Kalka
The phenomenon of liquid-liquid phase separation (LLPS) through multivalent protein-protein, protein-RNA, RNA-RNA interactions leads to the formation of bimolecular condensates, the so-called membraneless organelles present in the cytoplasm, nucleus and on the cell membrane. The resulting condensates play an important role in cellular processes, including the regulation of transcription, chromatin organization and intracellular signaling. The latest studies indicate that the LLPS phenomenon may be of particular importance for the development of neurodegenerative and neoplastic diseases, thus constituting a new therapeutic target. The aim of the study is to test the ability of human galectins to form liquid-liquid phases.
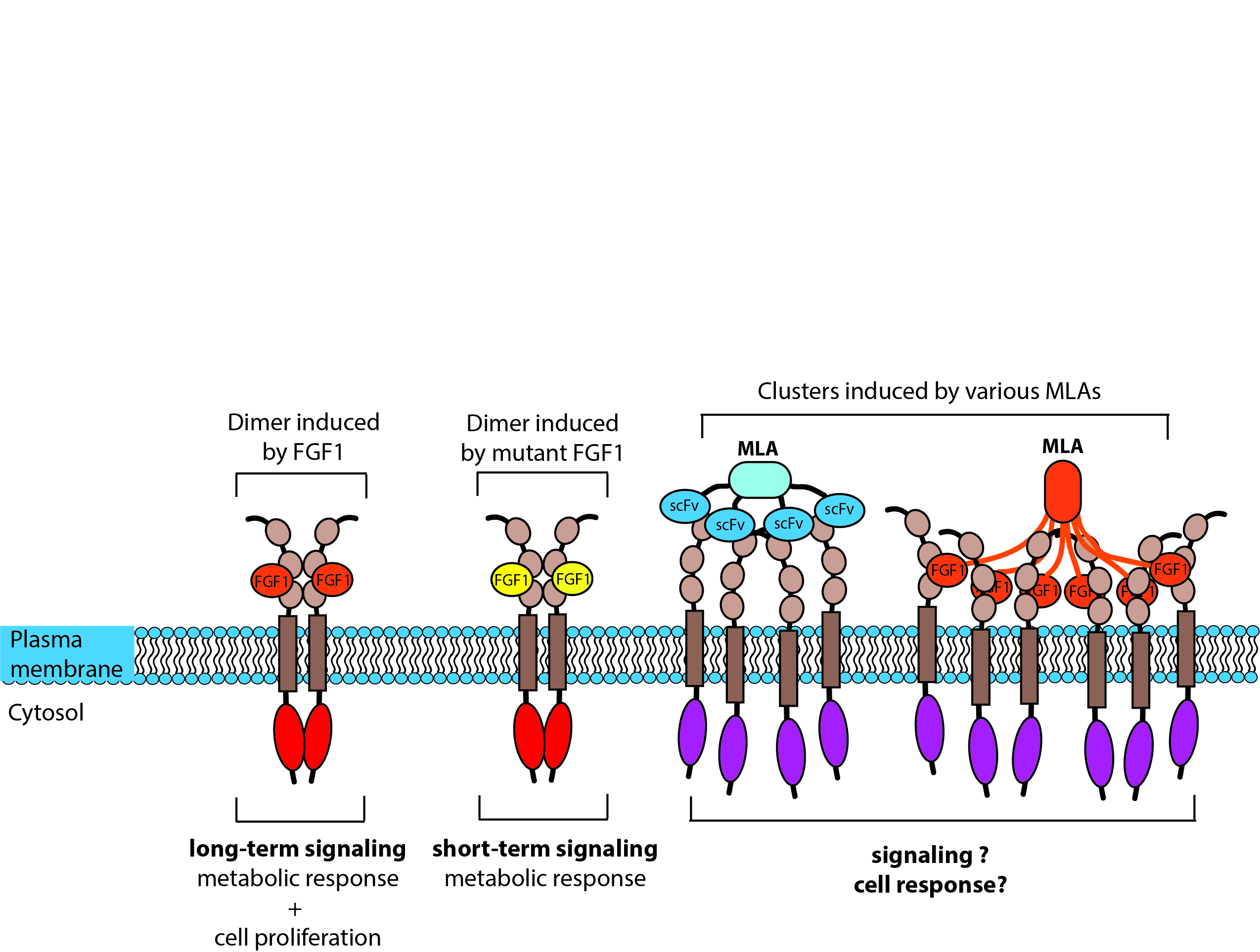
Control of FGFR1 spatial arrangement by multimeric ligands
Foundation for Polish Science FIRST TEAM Nr. POIR.04.04.00-00-43B2/17-00, PI: prof. dr hab. Łukasz Opaliński
The spatial organization of plasma membrane proteins can be achieved either through intracellular mechanisms (e.g. cytoskeleton, scaffolding proteins) or by using external mechanisms such as specific ligands. There are several examples showing that changes in the spatial organization of membrane proteins modulate their function. FGFR1 clustering has not been studied so far and it is not known how enforcing FGFR1 in oligomeric assemblies with different architectures will affect the function of FGFR1 in signal transduction, cell survival, migration and receptor trafficking. Since FGFR1 is often overexpressed by various types of cancers, designing multimeric ligands that enhance FGFR1 internalization may provide novel, highly effective targeting molecules for selective chemotherapy. The duration of FGFR1 signaling dictates the cellular outcome (metabolic vs. mitogenic response). Control of the spatial arrangement of FGFR1 may result in modulation of the cellular response and thus could potentially be used in the treatment of type 2 diabetes or tissue regeneration.
The overall goal of the project is to elucidate how clustering of FGFR1 into oligomers with different architectures on the cell surface will affect the function and cellular trafficking of the receptor. We hypothesize that the induction of different FGFR1 oligomeric structures will modulate FGFR1 activity and its internalization.

Molecular Basis of Internalization of Antibodies Directed Against Fibroblast Growth Factor Receptors (FGF)
NCN Nr 2015/16/S/NZ3/00363 (2015-2018); PI: prof. dr hab. Łukasz Opaliński
The aim of this project is to understand the molecular basis of selective internalization of antibodies directed against FGF receptors. Using specific endocytosis inhibitors, we will identify proteins interacting with the FGF receptor during the formation of the endocytic complex. Using a library of antibodies that bind to different extracellular regions of FGF receptors, we will investigate whether binding of FGFR by these antibodies affects the assembly of the endocytic complex. In this project, we will also determine whether extracellular regions of the FGF receptor can regulate endocytosis of the FGFR-antibody complex and determine how FGFR transmits the signal initiating endocytosis across the cell membrane. Using antibodies that bind to FGF receptors with different affinities, we will determine whether internalization of the antibody-FGFR complex is dependent on the energy of the antibody-FGFR interaction. We will also investigate whether antibodies can induce FGFR endocytosis by themselves or whether growth factor binding is additionally required for internalization of the antibody-FGFR complex. Finally, we will investigate whether antibody size affects the efficiency or the mechanism of internalization of the antibody-FGFR complex.
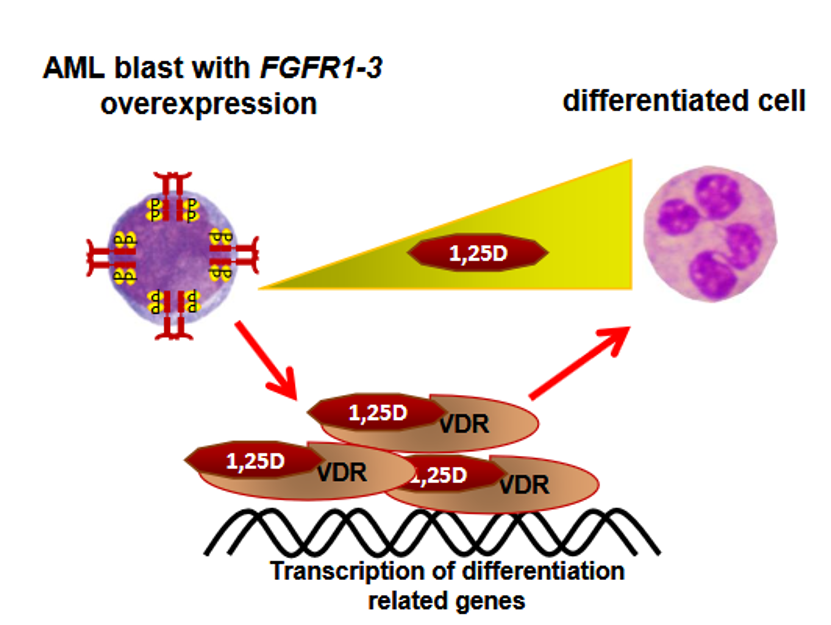
Interaction of signaling pathways from fibroblast growth factor receptors and the vitamin D receptor in the development of blood cancers
NCN Nr Nr 2016/23/B/NZ5/00065 (2017-2021); PI: prof. dr hab. Ewa Marcinkowska
Acute myeloid leukemia is a malignant blood disease that affects predominantly the elderly. Untreated, it has a very fast and severe course. Since it is a blood cell disease, the symptoms are related to functions of blood cells. It is accompanied by anemia, coagulation disorders and impaired immunity. Mutations in hematopoietic cells are at the root of this disease. About 200 different mutations that occur in patients with acute myeloid leukemia have been identified. Some of these mutations affect the genes which encode cell receptors for fibroblast growth factors, named FGFRs.
The treatment of acute myeloid leukemia consists of aggressive chemotherapy, followed by a transplantation of hematopoietic stem cells. Both procedures are too harsh for elderly patients, therefore there is a need for other, gentler treatments, which either can eliminate the disease, or let to live with it in a relative comfort. There is one subtype of acute myeloid leukemia, named acute promyelocytic leukemia, for which so-called targeted therapy has been found. This therapy, which directly targets the mutated protein with retinoic acid, is effective in nearly all patients with this subtype.
In our research, we focused on those subtypes in which the mutations affect FGFRs. We found that leukemic cells which have too many FGFRs can be induced (differentiated) to resemble mature immune cells, using active form of vitamin D and an inhibitor of one cell signalling pathway, named fludarabine. We believe that our observations are worth testing in mouse models, to verify if this would be a good targeted therapy for selected patients with acute myeloid leukemia.

Cooperation of the vitamin D receptor (VDR) with retinoic acid receptors (RAR) in the process of hematopoiesis
NCN Nr Nr 2015/17/B/NZ4/02632 (2017-2021); PI: prof. dr hab. Ewa Marcinkowska
Vitamin D is a prohormone produced photochemically in the skin from 7-dehydrocholesterol. It may be also obtained with diet or diet supplements. Vitamin D is metabolically activated in liver and in kidneys to an active hormone, which regulates calcium and phosphate homeostasis of the organism. Besides calcemic actions, an active form of vitamin D regulates immune system, however all details of this regulation have not been discovered so far. Vitamin D deficiency can cause not only rachitis or osteoporosis, but may also affect response to viruses. Recent reports point that deficient vitamin D status was associated with increased COVID-19 risk.
In our research project we studied the presence and the activity of the receptor for an active form of vitamin D in blood cells at all stages of their development in murine and human blood. Our studies have revealed that this receptor is more active in the cells at early steps of their development than in mature ones. The gene which encodes the receptor for vitamin D hormone is much more complex in humans than in mice, and it is regulated in a different way in both species. In murine blood cells this gene is strongly autoregulated by an active form of vitamin D, which does not occur in human blood cells. In contrast, in human blood cells this gene is regulated by an active metabolite of vitamin A, and such regulation does not occur in mice.
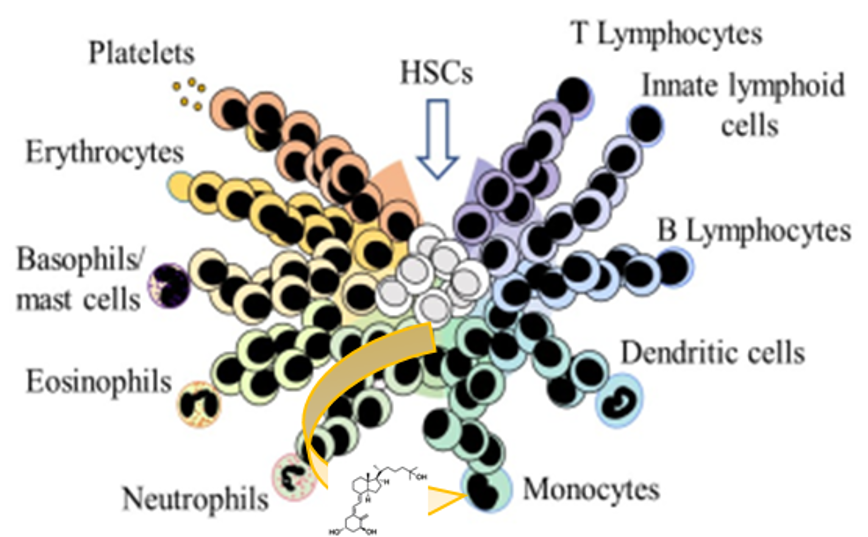
Decision-making within cells and differentiation entity therapies
MC-ITN (Marie Curie-Initial Training Networks); Call: FP7-PEOPLE-2012-ITN (2013-2017), PI: prof. dr hab. Ewa Marcinkowska
The overarching aims of DECIDE have been to: 1) contribute towards a better understanding of haematopoiesis and 2) generate new analogues of 1,25-dihydroxyvitamin D (1,25D) that have a reduced calcaemic action and are potent anti-cancer agents (termed vitamin D analogues; VDAs). Outcomes from the work have moved forward both of these areas.
1) Our viewpoint on haematopoiesis has moved away from a strict hierarchical scheme towards a pattern of haematopoietic stem cells/haematopoietic progenitor cells (HSC/HPC) behaviour that is much more flexible. The options revealed for HPC by in vitro assays might be viewed as clandestine options and HSC might be seen as less malleable in vivo. However, an important caveat is that steady state haematopoiesis is often studied and clandestine options could well be important when the system is stressed and there is an increased demand for a particular cell type. The extent to which the haematopoietic cytokines as presented by mesenchymal stem cells and the secretome of these cells play an instructive role during haematopoiesis requires further investigation.
2) Work in this project has led to the synthesis of new VDAs that have a substantially reduced calcaemic action and that are potent anticancer agents. This is important because the long term limitation for the use of 1,25D in cancer therapy has been the achievement of an effective therapeutic dose because of its calcaemic action. The new VDAs mean that a more effective dose of drug is likely to be achievable. The new VDAs arose from a comprehensive review of the structure and biological activities of existing VDAs. Beneficial modifications were identified and new chemistry was required to synthesise even newer VDAs. Two of the new analogues are substantially less calcaemic than 1,25D, as tested in mice, and they are a log fold more potent than 1,25D against acute myeloid leukaemia (AML) cell lines. The new VDAs bind effectively to the receptor for 1,25D (VDR). However, an important finding is that their enhanced anticancer action of VDAs does not correlate with their binding affinity or transactivation potency for VDR. Hence, their precise mode of action remains unclear.
https://www.birmingham.ac.uk/generic/decide
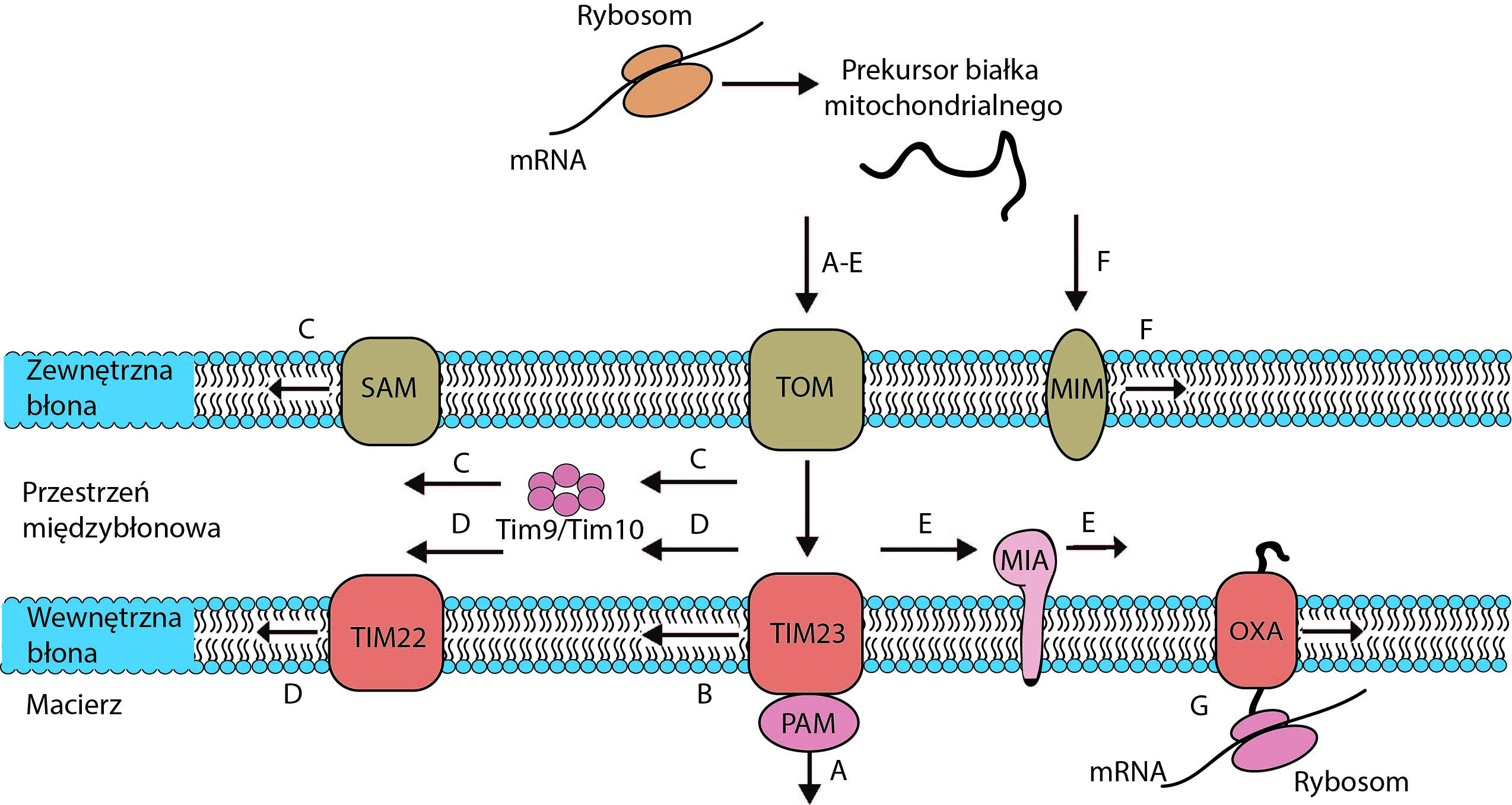
Mitochondrial protein biogenesis: transport of multi-spanning membrane proteins to mitochondria
EMBO LTF (EMBO) EMBO ALTF-948-2012 (2013-2015), PI-prof. dr hab. Łukasz Opaliński
Mitochondria are autonomous organelles that play a central role in cellular metabolism, ATP production, and the regulation of cell death by apoptosis. The vast majority of mitochondrial proteins are encoded by the nuclear genome, synthesized on cytosolic ribosomes, and delivered to the target organelle. Five major pathways for protein import into mitochondria have been characterized to date. Four of them can transport hydrophobic mitochondrial membrane proteins. To prevent aggregation of hydrophobic precursors in the cytosol and to ensure their efficient transport to mitochondrial membranes, precursors are directed to the organelle surface by cytosolic proteins. However, the composition of cytosolic forms of mitochondrial membrane protein transport and the mechanism by which precursors reach mitochondria are largely unknown. The aim of this project is to identify and characterize cytosolic forms of transport of different types of mitochondrial precursor proteins and their interactions with mitochondria.